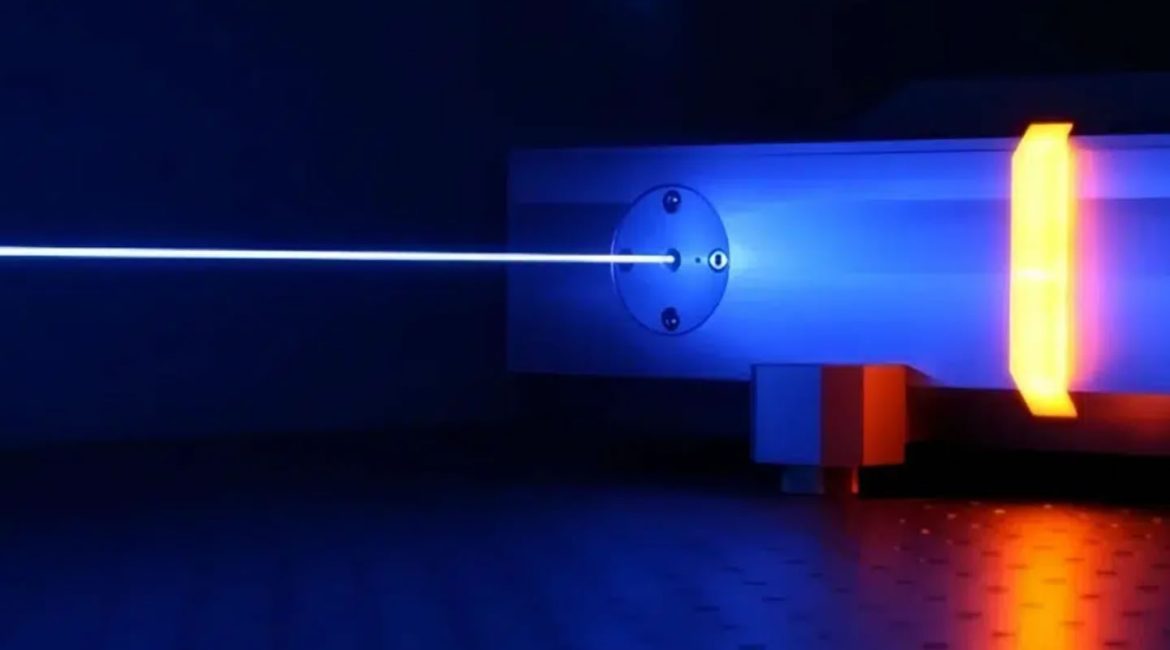
The CO2 laser tube, also known as a carbon dioxide laser, is a leading high-power laser source. It is widely used and highly mature in modern industry. This device acts as an efficient “light energy converter.” It transforms electrical energy into a powerful, focused infrared beam. Furthermore, this beam finds extensive use. Applications include cutting, engraving, welding, and marking across various industrial sectors.
I. Historical Origins and Core Industrial Status
C.K.N. Patel of Bell Labs invented the CO2 laser in 1964. Its advent significantly boosted laser technology in industrial applications. This laser achieved its vital status for a key reason. Its wavelength, about 10.6 micrometers, perfectly matches the absorption of many non-metallic materials. Examples include wood, plastics, paper, and fabrics. It also matches certain metals.
Today, you will find CO2 laser tubes everywhere. They power heavy machinery cutting thick steel plates in large automobile factories. They also operate equipment engraving wood and acrylic in small workshops. These tubes remain an unshakeable core force in laser processing. This is due to their high efficiency, high power, and relatively low operating costs.
II. Working Principle: Gas Discharge Ignites the “Glow”
How does this “light energy converter” work? The CO2 laser tube operates through a simple principle. It generates powerful light by “igniting” a gas mixture. Think of it as a magic trick.
- Core Materials: The “Magic Gases”
The CO2 laser tube does not contain only pure CO2 gas. Instead, it holds a carefully formulated gas mixture. This mixture typically includes three main components:
· Carbon Dioxide (CO2): This is the primary molecule. It generates the laser light.
· Nitrogen (N2): It acts as an “energy cheerleader.” Electrical energy easily excites nitrogen molecules. Then, they efficiently transfer energy to CO2 molecules. This helps CO2 molecules quickly reach an excited state (higher energy level).
· Helium (He): This gas functions as a “coolant” and “stabilizer.” It helps remove excess heat from CO2 molecules after photon emission. This allows CO2 molecules to quickly return to their ground state (lower energy level). Consequently, this maintains continuous, stable laser output.
- Excitation Process: “High-Voltage Ignition”
We must supply energy to these gas molecules to make them emit light. High-voltage discharge performs this task. Electrodes typically sit at both ends of the CO2 laser tube. When external high voltage (DC or RF) applies, ionization discharge occurs. This happens within the gas mixture. It generates high-speed electrons.
These electrons act like energy “bullets.” They rapidly collide with nitrogen (N2) molecules. The excited nitrogen molecules, now high in energy, then collide with CO2 molecules. They precisely transfer energy. This causes the CO2 molecules to jump to a higher energy level (excited state).
- Laser Generation: “Light Energy Amplification”
High-energy CO2 molecules are unstable. They spontaneously fall to a lower energy level. During this transition, they release excess energy. These are the photons we need. These photons travel back and forth between the reflective mirrors. These mirrors are at both ends of the CO2 laser tube. This space is known as the “resonant cavity.” They continuously “induce” other excited CO2 molecules. This stimulates them to release identical photons.
This process resembles an “avalanche-like” amplification of photons. Ultimately, the photon beam’s energy amplifies sufficiently. It then passes through the partially transmissive output mirror. This forms a powerful, concentrated, and directional light beam. This is the laser we observe.
In summary, the CO2 laser tube uses electrical energy. It drives gas discharge. It converts gas molecule energy into a high-energy photon beam. This achieves a miraculous transformation from “gas” to “light.” Thus, it provides modern industry with a powerful and precise tool.